About the project
Every year, thousands of new chemicals enter the market, adding to the vast array already in use. However, only a small portion of these chemicals are subjected to thorough assessments of their toxicity, leaving many potential health risks unexplored, particularly in cardiovascular safety.
The ALTERNATIVE project addresses this gap by developing an innovative platform that enhances the assessment of chemical cardiotoxicity. This new approach enables regulators and industry stakeholders to identify and manage cardiotoxic risks more effectively, reducing the reliance on animal testing and improving safety.
Description of success
ALTERNATIVE has developed a comprehensive three-step approach to improving the regulatory assessment of cardiotoxicity:
1. Identify current regulatory limitations. A review of current regulations uncovered significant gaps in cardiotoxicity assessment. This included the limited relevance and low prognostic power of both animal-based and artificial methods, and the overlooked impact on vulnerable populations, such as the elderly, as well as the effects of chemical mixtures.
2. Collect and integrate multiple lines of evidence for heart failure caused by environmental chemicals using the AOP framework. The AOP framework was employed to analyse the results of 17 epidemiological studies involving over 45,000 participants, and over 360 toxicological studies, and showed a significant link between environmental chemicals and cardiovascular diseases. This led to the development of a comprehensive AOP network that describes the pathways from chemical exposure to cardiac dysfunction.
3. Draft an Integrated Approach to Testing and Assessment (IATA) for cardiotoxicity. Using the established AOP network, an IATA was developed for cardiotoxicity within a future Next Generation Risk Assessment (NGRA) approach. This approach combines existing non-animal methods and methods developed by the ALTERNATIVE project to provide a comprehensive assessment framework to identify and assess the potential cardiotoxicity of chemicals.
Highlights
Comprehensive AOP network that maps the various mechanisms by which environmental chemicals affect cardiovascular health, from which to base human-relevant cardiotoxicity assessments.
A systematic review epidemiology and toxicology to show the link between chemical exposure and cardiovascular disease, creating awareness among regulators and the public.
Innovative testing approach (IATA) that incorporates advanced non-animal methods, increasing accuracy of cardiotoxicity assessment while reducing the need for animal testing.
Outputs
The ALTERNATIVE project focused on several tasks and produced several written outputs.
Firstly, current regulatory limitations were identified, and the following outputs were produced:
Position paper “Cardiotoxicity of chemicals: current regulatory guidelines, knowledge gaps, and needs”
White paper “Regulation of chemical exposures and cardiac health: Current gaps and future solutions”.
Secondly, the project focused on collecting and integrating multiple lines of evidence for heart failure caused by environmental chemicals using the AOP framework. The following outputs were produced:
Publication “Pollutant exposure and myocardial injury: Protocol and progress report for a toxicological systematic mapping review”
Publication “Artificial intelligence and machine learning methods to evaluate cardiotoxicity following the adverse outcome pathway frameworks”.
Lastly, an IATA for cardiotoxicity was drafted, which produced an output called “General structure of IATA for cardiotoxicity”.
Impact
The ALTERNATIVE project marks a significant advancement in our understanding of how environmental chemicals impact cardiovascular health. The integration of modern toxicological research into regulatory frameworks improves our ability to assess the risks that environmental chemicals have on cardiovascular health.
The shift towards non-animal testing methods, particularly through approaches like Next Generation Risk Assessment (NGRA), promotes compliance with ethical standards while also increasing the relevance of findings to human health. This is due to the NGRA's use of existing non-animal methods and innovative techniques developed through initiatives like the ALTERNATIVE project. These frameworks offer a comprehensive assessment to identify and evaluate the potential cardiotoxicity of chemicals, providing results that are more directly applicable to human biology and health. By leveraging the AOP framework, non-animal testing methods and NGRA, the innovative approach to cardiotoxicity assessment developed by ALTERNATIVE offers a more precise and human-centric approach to evaluating cardiotoxicity. As a result, this work is set to significantly enhance cardiovascular safety, providing regulators and industry with the tools to better protect the public against the risks posed by environmental chemicals.
Lessons
Value of integrated evidence: Combining epidemiological and toxicological data enhances the completeness and accuracy of chemical risk assessments and demonstrates the importance of a multi-faceted approach.
Advantages of non-animal methods: Shifting to non-animal testing methods using the NGRA approach improves relevance to human health and aligns with ethical standards but requires careful planning to ensure regulatory acceptance.
Cross-disciplinary collaboration: Effective teamwork across various scientific and regulatory disciplines is crucial for developing comprehensive assessment frameworks within the transition to NGRA.
Importance of communication: Regular engagement with the scientific community, regulators and the public is essential to promote understanding and acceptance of innovative methodologies.
Other information
Project ALTERNATIVE is set to expand its impact by developing case studies that demonstrate the practical applications of its cardiotoxicity IATA, highlighting its effectiveness in real-world scenarios. It aims to integrate this approach with broader (NGRA concepts to establish a unified framework for chemical risk assessment.
Additionally, it plans to broaden its IATA frameworks to include more toxicological endpoints and environmental conditions, increasing their applicability and relevance. Strengthening collaborations with regulatory bodies, industry stakeholders and academic institutions will also be crucial to promoting the adoption and ongoing refinement of the project’s methodologies.

Figure 1: Regulatory need for cardiotoxicity assessment. Source: ALTERNATIVE
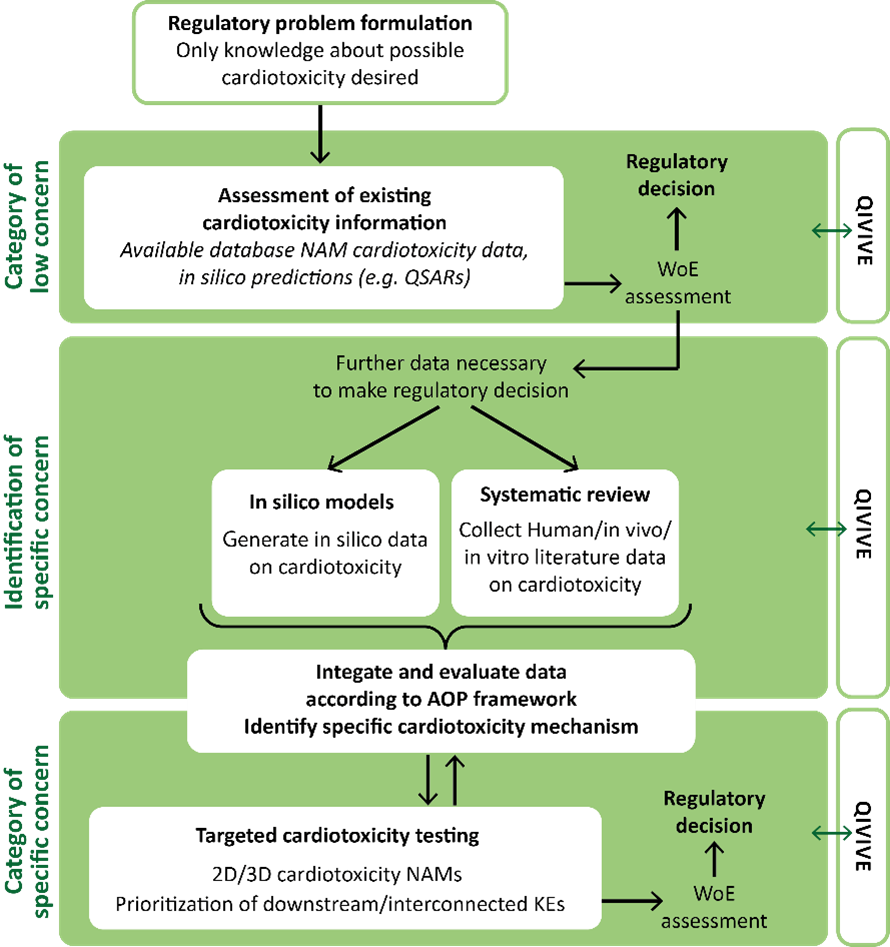
Figure 2: Proposed Cardiotoxicity IATA Source: ALTERNATIVE

